
CHALLENGE: Give people with lymphedema an effective and comfortable way to care for themselves at home.
SERVICES: Insights, Design, Engineering, Prototyping, Sourcing, Manufacturing
AWARDS / PRESS: Medgadget; Star Tribune; Women's Health Mag
SUCCESS: Designed first-of-its-kind pneumatic head and neck garment
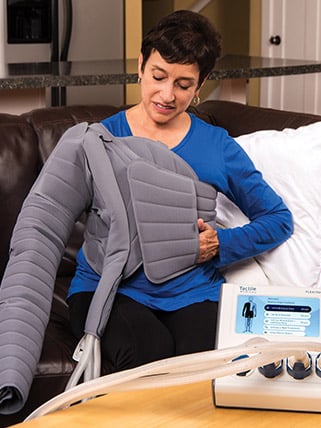
Above: The Flexitouch Plus display was improved by increasing the screen size, including color for improved usability, and the addition of soft-touch keys.
Tactile Medical is a leader in developing and marketing at-home therapy devices that treat lymphedema, an impairment to the lymphatic system that results in painful, disfiguring swelling. Untreated lymphedema can lead to irreversible conditions like the formation of fibrotic tissue and recurring episodes of cellulitis. Though sometimes inherited, lymphedema is most often the result of a disruption of the lymphatic system, caused by vascular disease, cancer treatment, or obesity. An estimated five million people in the U.S., and 250 million people worldwide, are affected.
Tactile Medical manufactures pneumatic compression devices that allow lymphedema patients to self-manage at home. The company approached Nottingham Spirk to develop a first-to-market pneumatic compression garment for use on the head and neck and to create the next generation Flexitouch Plus System. We were asked to improve the ease of donning and doffing the various pneumatic compression garments and to re-design the system controller without increasing cost.

We worked closely with Tactile Medical’s staff clinicians to understand how pneumatic compression garments mimic manual lymph drainage (MLD) applied by a certified therapist to stimulate the lymphatic system and increase the uptake of lymph fluid. Precise fit of the garments is important to deliver the skin stretch necessary for effective therapy. We hired fit models of varying sizes to help us design for a wide range of user body types, then worked with the garment supplier to create functional soft good, pneumatic prototypes for extensive testing for ease of use, comfort and durability.
We completely redesigned the controller’s user interface and designed a bilateral adapter that now allows the patient to conduct treatment on both legs at the same time, where the previous Flexitouch could not. This was a significant engineering challenge and led to successful IP generation. We also improved the display to a 7-inch color screen and added soft-touch keys. By carefully sourcing new materials, we were able to hold the manufacturing cost of the new Flexitouch Plus controller to within 1 percent of the previous Flexitouch controller, which helped maintain Tactile Medical’s profitability.
The first-of-its-kind head and neck garment (which works with Tactile’s installed base of Flexitouch controllers), the Flexitouch Plus controller and new easier to use garments are all FDA cleared.

A CONNECTED CONSUMER MEDICAL DEVICE TO IMPROVE MEDICATION ADHERENCE RATES

BASSINET THAT SWIVELS TO PROVIDE A SAFE AND EASY CO-SLEEPING EXPERIENCE

Learn how Nottingham Spirk can help you design and commercialize your next product innovation.
2200 Overlook Road
Cleveland, Ohio
44106
© 2023 Nottingham Spirk All rights reserved.